Mechanical Ventilatory Support
Mechanical ventilatory Support is a therapeutic method that is used to assist or replace spontaneous breathing. The primary indication for initiation of mechanical ventilation is respiratory failure, of which there are two basic types: hypoxemic respiratory failure, which is present when arterial O2 saturation (SaO2) <90% occurs despite an increased inspired O2 fraction, and hypercarbic respiratory failure, which is characterized by arterial PCO2 values >50 mmHg. When it is chronic, neither of the two types is obligatorily treated with mechanical ventilation, but when acute, mechanical ventilation may be lifesaving.
Indications
The most common reasons for instituting mechanical ventilation are acute respiratory failure with hypoxemia (acute respiratory distress syndrome, heart failure with pulmonary edema, pneumonia, sepsis, complications of surgery and trauma), which accounts for ~65% of all ventilated cases, followed by causes of hypercarbic ventilatory failure such as coma (15%), exacerbations of chronic obstructive pulmonary disease (13%), and neuromuscular diseases (5%). The primary objectives of mechanical ventilation are to decrease the work of breathing, thus avoiding respiratory muscle fatigue, and to reverse life-threatening hypoxemia and progressive respiratory acidosis.
In some cases, mechanical ventilation is used as an adjunct to other forms of therapy, such as its use in reducing cerebral blood flow in patients with increased intracranial pressure. Mechanical ventilation also is used frequently in conjunction with endotracheal intubation to prevent aspiration of gastric contents in otherwise unstable patients during gastric lavage for suspected drug overdose or during gastrointestinal endoscopy. In critically ill patients, intubation and mechanical ventilation may be indicated before essential diagnostic or therapeutic studies if it appears that respiratory failure may occur during those maneuvers.
Types of Mechanical Ventilation
In its broadest sense, there are two distinct methods for ventilating patients: noninvasive ventilation (NIV) and invasive ventilation or conventional mechanical ventilation (MV).
Noninvasive Ventilation
Noninvasive ventilation has been gaining more acceptance because it is effective in certain conditions, such as acute or chronic respiratory failure, and is associated with fewer complications, namely, pneumonia and tracheolaryngeal trauma. Noninvasive ventilation usually is provided by using a tight-fitting face mask or nasal mask similar to the masks traditionally used for treatment of sleep apnea. Noninvasive ventilation has proved highly effective in patients with respiratory failure from acute exacerbations of chronic obstructive pulmonary disease and is most frequently implemented by using bilevel positive airway pressure ventilation or pressure support ventilation. In both of these modes, a preset positive pressure is applied during inspiration and a lower pressure is applied during expiration at the mask. Both modes are well tolerated by a conscious patient and optimize patient-ventilator synchrony. The major limitation to its widespread application has been patient intolerance because the tight-fitting mask required for NIV can cause both physical and emotional discomfort. In addition, NIV has had limited success in patients with acute hypoxemic respiratory failure, for whom endotracheal intubation and conventional MV remain the ventilatory method of choice.
The most important group of patients who benefit from a trial of NIV are those with acute exacerbations of chronic obstructive pulmonary disease (COPD) leading to respiratory acidosis (pH <7.35). Experience from several well-conducted randomized trials has shown that in patients with ventilatory failure characterized by blood pH levels between 7.25 and 7.35, NIV is associated with low failure rates (15–20%) and good outcomes (intubation rate, length of stay in intensive care, and in some series mortality rates). In more severely ill patients with pH <7.25, the rate of NIV failure is inversely related to the severity of respiratory acidosis, with greater failure as the pH decreases. In patients with milder acidosis (pH >7.35), NIV is not better than conventional therapy that includes controlled oxygen delivery and pharmacotherapy for exacerbations of COPD (systemic corticosteroids, bronchodilators, and, if needed, antibiotics).
Despite its benign outcomes, NIV is not useful in the majority of cases of respiratory failure and is contraindicated in patients with the conditions listed in Table S-1. Experience shows that NIV can delay lifesaving ventilatory support in those cases and actually results in aspiration or hypoventilation. Once NIV is initiated, patients should be monitored; a reduction in respiratory frequency and a decrease in the use of accessory muscles (scalene, sternomastoid, and intercostals) are good clinical indicators of adequate therapeutic benefit. Arterial blood gases should be obtained at least within hours of the initiation of therapy to ensure that NIV is having the desired effect and that it is safe to continue its application. Lack of benefit within that time frame should alert one to the possible need for conventional MV.
| Table S–1. Contraindications for Noninvasive Ventilation |
| Cardiac or respiratory arrest |
| Severe encephalopathy |
| Severe gastrointestinal bleed |
| Hemodynamic instability |
| Unstable angina and myocardial infarction |
| Facial surgery or trauma |
| Upper airway obstruction |
| High-risk aspiration and/or inability to protect airways |
| Inability to clear secretions |
Conventional Mechanical Ventilation
Conventional mechanical ventilation is implemented once a cuffed tube is inserted into the trachea to allow conditioned gas (warmed, oxygenated, and humidified) to be delivered to the airways and lungs at pressures above atmospheric pressure. Great care has to be taken during the act of intubation to avoid brain-damaging hypoxia. In some patients, intubation can be achieved without added sedation. In most patients, the administration of mild sedation may help facilitate the procedure. Opiates and benzodiazepines are good choices but can have a deleterious effect on hemodynamics in patients with depressed cardiac function or low systemic vascular resistance. Morphine can promote histamine release from tissue mast cells and may worsen bronchospasm in patients with asthma; fentanyl, sufentanil, and alfentanil are acceptable alternatives. Ketamine may increase systemic arterial pressure and has been associated with hallucinatory responses; it should be used with caution in patients with hypertensive crisis or a history of psychiatric disorders. Newer agents such as etomidate and propofol have been used for both induction and maintenance of anesthesia in ventilated patients. They are shorter-acting, and etomidate has fewer adverse hemodynamic effects, but both agents are significantly more expensive than older agents. Great care must be taken to avoid the use of neuromuscular paralysis during intubation; in particular, the use of agents whose mechanism of action includes depolarization at the neuromuscular junction, such as succinylcholine chloride, should be avoided in patients with renal failure, tumor lysis syndrome, crush injuries, medical conditions associated with elevated serum potassium levels, and muscular dystrophy syndromes.
Principles of Mechanical Ventilation
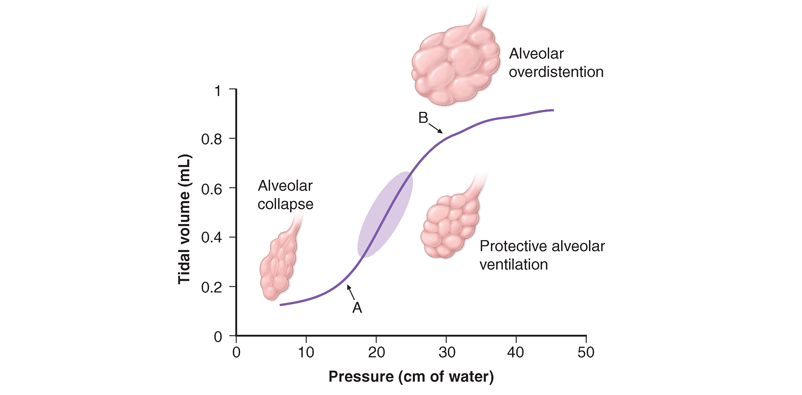
Once the patient has been intubated, the basic principles of applying MV are to optimize oxygenation while avoiding overstretch and collapse/rerecruitment ventilator-induced lung injury (VILI). This concept, which is illustrated in Fig. 269-1, has gained acceptance because of important empirical and experimental evidence linking high airway pressures and volumes and overstretching the lung with collapse/rerecruitment with poor outcomes. Although normalization of pH through elimination of CO2 is desirable, the risk of lung damage associated with the large volume and high pressures needed to achieve this goal has led to the acceptance of permissive hypercapnia. This approach has been found to be well tolerated when care is taken to avoid excess acidosis by pH buffering.
Modes of Ventilation
Mode refers to the manner in which ventilator breaths are triggered, cycled, and limited. The trigger, either an inspiratory effort or a time-based signal, defines what the ventilator senses to initiate an assisted breath. Cycle refers to the factors that determine the end of inspiration. For example, in volume-cycled ventilation, inspiration ends when a specific tidal volume is delivered. Other types of cycling include pressure cycling and time cycling. The limiting factors are operator-specified values, such as airway pressure, that are monitored by transducers internal to the ventilator circuit throughout the respiratory cycle; if the specified values are exceeded, inspiratory flow is terminated, and the ventilator circuit is vented to atmospheric pressure or the specified pressure at the end of expiration [positive end-expiratory pressure (PEEP)]. Most patients are ventilated with assist control ventilation, intermittent mandatory ventilation, or pressure-support ventilation, with the latter two modes often used simultaneously (Table S-2).
Table S–2. Characteristics of the Most Commonly Used Forms of Mechanical Ventilation
| Ventilatory Mode | Variables Set by User (Independent) | Variables Monitored by User (Dependent) | Trigger Cycle Limit | Advantages | Disadvantages |
| ACMV (assist control ventilation) | Tidal volume Ventilator rate FIO2 PEEP level Pressure limit | Peak, mean, and plateau airway pressures VE ABG I/E ratio | Patient effort Timer Pressure limit | Patient control Guaranteed ventilation | Potential to hyperventilate Barotrauma and volume trauma Every effective breath generates a ventilator volume |
| IMV (intermittent mandatory ventilation) | Tidal volume Mandatory Ventilator Rate FIO2 PEEP level Pressure limit Between breaths patients can breathe spontaneously | Peak, mean, and plateau airway pressures VE ABG I/E ratio | Patient effort Timer Pressure limit | Patient control Comfort from spontaneous breaths Guaranteed ventilation | Potential dysynchrony May result in hypoventilation |
| PSV (pressure support ventilation) | Inspiratory pressure level FIO2 PEEP Pressure limit | Tidal volume Respiratory rate VE ABG | Pressure limit Inspiratory flow | Patient control Comfort Assures synchrony | No timer backup May result in hypoventilation |
| NIV (noninvasive ventilation) | Inspiratory and expiratory pressure level FIO2 | Tidal volume Respiratory rate VE ABG | Pressure limit Inspiratory flow | Patient control | Mask interface may cause discomfort and facial bruising Leaks are common Hypoventilation |
Assist Control Ventilation (ACMV)
This is the most widely used mode of ventilation. In this mode, an inspiratory cycle is initiated either by the patient’s inspiratory effort or, if none is detected within a specified time window, by a timer signal within the ventilator. Every breath delivered, whether patient- or timer-triggered, consists of the operator-specified tidal volume. Ventilatory rate is determined either by the patient or by the operator-specified backup rate, whichever is of higher frequency. ACMV commonly is used for initiation of mechanical ventilation because it ensures a backup minute ventilation in the absence of an intact respiratory drive and allows for synchronization of the ventilator cycle with the patient’s inspiratory effort.
Problems can arise when ACMV is used in patients with tachypnea due to nonrespiratory or nonmetabolic factors, such as anxiety, pain, and airway irritation. Respiratory alkalemia may develop and trigger myoclonus or seizures. Dynamic hyperinflation leading to increased intrathoracic pressures (so-called auto-PEEP) may occur if the patient’s respiratory mechanics are such that inadequate time is available for complete exhalation between inspiratory cycles. Auto-PEEP can limit venous return, decrease cardiac output, and increase airway pressures, predisposing to barotrauma.
Intermittent Mandatory Ventilation (IMV)
With this mode, the operator sets the number of mandatory breaths of fixed volume to be delivered by the ventilator; between those breaths, the patient can breathe spontaneously. In the most frequently used synchronized mode (SIMV), mandatory breaths are delivered in synchrony with the patient’s inspiratory efforts at a frequency determined by the operator. If the patient fails to initiate a breath, the ventilator delivers a fixed-tidal-volume breath and resets the internal timer for the next inspiratory cycle. SIMV differs from ACMV in that only the preset number of breaths is ventilator-assisted.
SIMV allows patients with an intact respiratory drive to exercise inspiratory muscles between assisted breaths, making it useful for both supporting and weaning intubated patients. SIMV may be difficult to use in patients with tachypnea because they may attempt to exhale during the ventilator-programmed inspiratory cycle. When this occurs, the airway pressure may exceed the inspiratory pressure limit, the ventilator-assisted breath will be aborted, and minute volume may drop below that programmed by the operator. In this setting, if the tachypnea is in response to respiratory or metabolic acidosis, a change in ACMV will increase minute ventilation and help normalize the pH while the underlying process is further evaluated and treated.
Pressure-Support Ventilation (PSV)
This form of ventilation is patient-triggered, flow-cycled, and pressure-limited. It provides graded assistance and differs from the other two modes in that the operator sets the pressure level (rather than the volume) to augment every spontaneous respiratory effort. The level of pressure is adjusted by observing the patient’s respiratory frequency. During PSV, the inspiration is terminated when inspiratory airflow falls below a certain level; in most ventilators, this flow rate cannot be adjusted by the operator. When PSV is used, patients receive ventilator assistance only when the ventilator detects an inspiratory effort. PSV frequently is used in combination with SIMV to ensure volume-cycled backup for patients whose respiratory drive is depressed. PSV frequently is well tolerated by most patients who are being weaned; PSV parameters can be set to provide full or nearly full ventilatory support and can be withdrawn to load the respiratory muscles gradually.
There are other modes of ventilation, and each has its own acronym, making it very difficult to understand for those unfamiliar with the terms. All these modes are modifications of the manner and duration in which pressure is applied to the airway and lungs and of the interaction between the mechanical assistance provided by the ventilator and the patient’s respiratory effort. Although their use in acute respiratory failure is limited, the following have been used with varying levels of enthusiasm and adoption.
Pressure-Control Ventilation (PCV)
This form of ventilation is time-triggered, time-cycled, and pressure-limited. During the inspiratory phase, a specified pressure is imposed at the airway opening throughout inspiration. Since the inspiratory airway pressure is specified by the operator, tidal volume and inspiratory flow rate are dependent, rather than independent, variables and are not operator-specified. PCV is the preferred mode of ventilation for patients in whom it is desirable to regulate peak airway pressures, such as those with preexisting barotrauma, and postoperative thoracic surgical patients, in whom the shear forces across a fresh suture line should be limited. When PCV is used, minute ventilation and tidal volume must be monitored closely; minute ventilation is altered through changes in rate or in the pressure-control value, which changes tidal volume.
Inverse Ratio Ventilation (IRV)
This mode of ventilation is a variant of PCV that incorporates the use of a prolonged inspiratory time with the appropriate shortening of the expiratory time. It has been used in patients with severe hypoxemic respiratory failure. This approach increases mean distending pressures without increasing peak airway pressures. It is thought to work in conjunction with PEEP to open collapsed alveoli and improve oxygenation, although there are no conclusive data showing that IRV improves outcomes in clinical trials.
Continuous Positive Airway Pressure (CPAP)
This is not a true support mode of ventilation because all ventilation occurs through the patient’s spontaneous efforts. The ventilator provides fresh gas to the breathing circuit with each inspiration and sets the circuit to a constant, operator-specified pressure. CPAP is used to assess extubation potential in patients who have been effectively weaned and require little ventilator support and patients with intact respiratory system function who require an endotracheal tube for airway protection.
Nonconventional Ventilatory Strategies
Several nonconventional ventilator strategies have been evaluated for their ability to improve oxygenation and reduce mortality rates in patients with advanced hypoxemic respiratory failure. These strategies include high-frequency oscillatory ventilation (HFOV), airway pressure release ventilation (APRV), extracorporeal membrane oxygenation (BCMO), and partial liquid ventilation (PLV) using perfluorocarbons. Although case reports and small uncontrolled cohort studies have shown benefit, randomized controlled trials have failed to demonstrate consistent improvements in outcome with any of these strategies. Currently, these approaches should be considered “salvage” techniques and considered for patients with hypoxemia refractory to conventional therapy. Prone positioning of patients with refractory hypoxemia has been explored because in theory it would tend to improve ventilation-perfusion matching. Although this is conceptually appealing and simple to implement, several randomized trial in patients with acute lung injury did not demonstrate a survival advantage with prone positioning despite demonstration of a transient physiologic benefit. The administration of nitric oxide (NO) gas, which has bronchodilator and pulmonary vasodilator effects when delivered through the airways and has been shown to improve arterial oxygenation in many patients with advanced hypoxemic respiratory failure, also failed to improve outcomes in patients with advanced hypoxemic respiratory failure.
Newer, promising strategies are intended to improve patient-ventilator synchrony, a major practical problem during MV. Currently, the more advanced new ventilators allow patients to trigger the ventilator with their own effort while also incorporating flow algorithms that allow termination of cycles once certain preset criteria are reached; this approach has greatly improved patient-ventilator synchrony and comfort. More recently, new modes of ventilation that synchronize not only the timing but also the levels of assistance to match the patient’s effort have been developed. Proportional assist ventilation (PAV) and neurally adjusted ventilatory assist ventilation (NAV) are two modes that are designed to deliver assisted breaths through algorithms incorporating not only pressure, volume, and time but also overall respiratory resistance and compliance in the case of PAV and neural activation of the diaphragm in the case of NAV. Although these modes result in better patient-ventilator synchrony, their practical use in the everyday management of patients on MV needs further study.
Protective Ventilatory Strategy
Whichever mode of MV is used, in acute respiratory failure the evidence from several important controlled trials indicates that the use of a protective ventilation approach guided by the principles outlined below and summarized in Fig. S-1 is safe and offers the best chance of a good outcome:
- Set a target tidal volume close to 6 mL/kg of ideal body weight.
- Prevent plateau pressure (static pressure in the airway at the end of inspiration) over 30 cmH2O.
- Use the lowest possible fraction of inspired oxygen (FIO2) to keep SaO2 ≥90%.
- Adjust the PEEP to maintain alveolar patency while preventing overdistention and closure/reopening.
With the application of these techniques, the mortality rate among patients with acute hypoxemic respiratory failure has decreased to ~30% from close to 50% a decade ago.
Patient Management
Once the patient has been stabilized with respect to gas exchange, definitive therapy for the underlying process responsible for respiratory failure is initiated. Subsequent modifications in ventilator therapy must be provided in parallel with changes in the patient’s clinical status. As improvement in respiratory function is noted, the first priority is to reduce the level of mechanical ventilator support. Patients on full ventilator support should be monitored frequently with the goal of switching to a mode that allows for weaning as soon as possible. Protocols and guidelines that can be applied by paramedical personnel when physicians are not readily available have proved to be of value in shortening ventilator and intensive care unit (ICU) time, with very good outcomes. Patients whose condition continues to deteriorate after ventilator support is initiated may require increased O2, PEEP, or one of the alternative modes of ventilation.
General Support during Ventilation
Patients started on mechanical ventilation usually require sedation and analgesia to maintain an acceptable level of comfort. Often, this consists of a combination of a benzodiazepine and an opiate administered intravenously. Medications commonly used for this purpose include lorazepam, midazolam, diazepam, morphine, and fentanyl. The use of oversedation must be avoided in the ICU. Indeed, recent trials evaluating the effect of daily interruption of sedation in patients with improved ventilatory status show that this results in shorter time on the ventilator and shorter ICU stay.
Immobilized patients in the ICU who are on mechanical ventilator support are at increased risk for deep venous thrombosis and decubitus ulcers. To prevent venous thrombosis, prophylaxis in the form of subcutaneous heparin and/or pneumatic compression boots is prescribed frequently. Fractionated low-molecular-weight heparin appears to be equally effective for this purpose. To help prevent decubitus ulcers, frequent changes in body position and the use of soft mattress overlays and air mattresses are employed. Prophylaxis against diffuse gastrointestinal mucosal injury is indicated for patients on MV. Histamine-receptor antagonists (H2-receptor antagonists), antacids, and cytoprotective agents such as Carafate (sucralfate) have all been used for this purpose and appear to be effective. Nutrition support by enteral feeding through either a nasogastric or an orogastric tube should be initiated and maintained whenever possible. Delayed gastric emptying is common in critically ill patients on sedative medications but often responds to promotility agents such as metoclopramide. Parenteral nutrition is an alternative to enteral nutrition in patients with severe gastrointestinal pathology who need prolonged MV.
Complications of Mechanical Ventilation
Endotracheal intubation and mechanical ventilation have direct and indirect effects on the lung and upper airways, the cardiovascular system, and the gastrointestinal system. Pulmonary complications include barotrauma, nosocomial pneumonia, oxygen toxicity, tracheal stenosis, and deconditioning of respiratory muscles. Barotrauma and volutrauma overdistend and disrupt lung tissue; may be clinically manifest by interstitial emphysema, pneumomediastinum, subcutaneous emphysema, or pneumothorax; and can result in the liberation of cytokines from overdistended tissues, further promoting tissue injury. Clinically significant pneumothorax requires tube thoracostomy. Intubated patients are at high risk for ventilator-associated pneumonia (VAP) as a result of aspiration from the upper airways through small leaks around the endotracheal tube cuff; the most common organisms responsible for this condition are Pseudomonas aeruginosa, enteric gram-negative rods, and Staphylococcus aureus. Because this condition is associated with high mortality rates, early initiation of empirical antibiotics directed against likely pathogens is recommended. Hypotension resulting from elevated intrathoracic pressures with decreased venous return is almost always responsive to intravascular volume repletion. In patients who are judged to have respiratory failure on the basis of alveolar edema but in whom the cardiac or pulmonary origin of the edema is unclear, hemodynamic monitoring with a pulmonary arterial catheter may be of value in helping to clarify the cause of the edema.
Gastrointestinal effects of positive-pressure ventilation include stress ulceration and mild to moderate cholestasis.
Weaning from Mechancal Ventilation
It is important to consider discontinuation of mechanical ventilation once the underlying respiratory disease begins to reverse. Although the predictive capacities of multiple clinical and physiologic variables have been explored, the consensus from a weaning task force includes the following recommendations: (1) lung injury is stable/resolving, (2) gas exchange is adequate with low PEEP/FIO2 (<8 cmH2O and FIO <0.5), (3) hemodynamic variables are stable (patient off vasopressors), and (4) patient is capable of initiating spontaneous breaths. This “screen” should be done at least daily. If the patient is deemed capable of beginning weaning, the recommendation of the task force is to perform a spontaneous breathing trial (SBT) because several randomized trials support the value of this approach (Fig. S-2). The SBT involves an integrated patient assessment during spontaneous breathing with little or no ventilator support. The SBT is usually implemented with a T-piece using 1–5 cmH2O CPAP or a T-piece with 5–7 cmH2O or PSV from the ventilator to offset the resistance from the endotracheal tube. Once it is determined that the patient can breath spontaneously, a decision must be made about the removal of the artificial airway; this should be done only when it is concluded that the patient has the ability to protect the airway, is able to cough and clear secretions, and is alert enough to follow commands. In addition, other factors must be taken into account, such as the possible difficulty in replacing the tube if that is anticipated. If upper airway difficulty is suspected, an evaluation using a “cuff leak” test (assessing the presence of air movement around a deflated endotracheal tube cuff) is supported by some internists. Despite the application of all of these methods, ~10–15% of extubated patients require reintubation. Several studies suggest that NIV can be used to avert reintubation; this has been particularly useful in patients with ventilatory failure secondary to COPD exacerbation. In this group, earlier extubation with the use of prophylactic NIV has shown good results. The use of NIV to facilitate weaning in other causes of respiratory failure is not currently indicated.

Prolonged Mechanical Ventilation and Tracheostomy
From 5 to 13% of patients on MV will go on to require prolonged MV (>21 days). In these patients, critical care personnel have to make a decision about whether and when to perform a tracheostomy. This decision is individually based on the risk and benefits of tracheostomy and prolonged intubation and the patient’s preferences and expected clinical outcomes. A tracheostomy is thought to be more comfortable, require less sedation, and provide a more secure airway, and it seems to reduce weaning time. However, tracheostomy carries the risk of complications, which occur in 5–40% of the procedures and include bleeding, cardiopulmonary arrest, hypoxia due to airway loss, structural damage, postoperative pneumothorax, pneumomediastinum, and wound infection. In patients with long-term tracheostomy, tracheal stenosis, granulation, and the erosion of the innominate artery are complex complications. It is generally agreed that if a patient is in need of MV for more than 10–14 days, a tracheostomy is indicated and should be planned under optimal conditions. Whether it is completed at the bedside or as an operative procedure depends on the local resources and experience. Some 5–10% of patients are deemed unable to wean in the ICU. These patients may benefit from transfer to special units where a multidisciplinary approach, including nutrition optimization, physical therapy with rehabilitation, and slower weaning methods, including SIMV with PSV, results in up to 30% successful weaning. Unfortunately, close to 2% of ventilated patients may ultimately remain unable to wean and become dependent on ventilatory support to maintain life. Most of these patients remain in chronic care institutions, although some who have strong social, economical, and family support may achieve a fulfilling life with home mechanical ventilation.
